Disclaimer: This post is for personal note, which I just so happen to be sharing. This post isn’t to encourage any unauthorized research or to spread misleading medical information. I’m simply theorizing in public about information I read from actual academics in order to find correlation in their research and to enhance my understanding of medicine, biology, chemistry, virology, etc. This paper is more of a set of notes, so I apologize for poor citations, improper citation format, etc. My view is that, I just want to connect the dots and figure out issues and formalities often get in the way.
Forwards: I’m not a medical or science professional, though I took some classes while in college. Yet, the more I read into the basics of RNA (AGCU), DNA (AGCT), proteins, enzymes, cell functions, etc., the more I seem to understand that fighting viruses (which are typically single strands of viral RNA code with a protein shell) is that you have to chemically “trick”, induce, or fortify healthy cells and trigger them in shutting off certain functions when exposed, but also turning on certain functions when exposed. These functions can range from preventing or discouraging endocytosis (when a cell “eats” a virus to incorporate into its body – cytoplasm, vacuoles, etc. – but the virus hacks the health DNA code), turning off or on certain proteins and enzymes, and triggering immune responses to seek and destroy the virus.
Also, in layman terms (meant for myself) vRNA (viral RNA) like most RNA has a phosphorous backbone and four chemicals, so it’s not entirely a “physical hack”, but more so a “chemical-secretion melody” that the virus “plays” or delivers (think of the chemicals as keys on a piano and when played make a tun) to infect a cell (re-tune or re-harmonize it) to encourage its replication, i.e., it tricks the healthy cell to stimulate ribosome production of viral proteins, enzymes, etc. The viruses are programmed to seek out certain proteins, antibodies, and/or enzymes in order to “pick” a cell’s lock or attract to it and lock in. Thus, if you’re shutting off and on things to fight viruses you might get residual physiological effects such as an immune system running on overdrive and attacking healthy bodily functions such as healthy renal (kidney) function.
I was studying viruses largely due to the Coronavirus dilemma and did some reading into anti-viral research that are using various methods such designing anti-viral mRNA (messenger RNA) which can possibly be loaded into nanoparticles such as poly [lactic-co-glycolic acid] nanoparticles; discouraging the attachment of sugar molecules to viral proteins (such as limiting OST complexes); understanding current medications such as Arbidol (used for influenza and studied regarding Zika – a distant cousin of Hepatitis C, West Nile, Dengue Fever, etc.) , NGI-1 (OST inhibitor), Remdesivir (Ebola), Apoptozole (Hsp70 inhibitor); utilizing protein inhibitors such as NSC 630668-R/1, VER-155008, MAL3–101, MKT-077, Pifithrin, and Apoptozole; preventing NS proteins which have roles in viral replication which includes NS1 (modulates host immunity), NS2A, NS2B, NS3 (protease), NS4A, NS4B, NS5 (polymerase); inhibiting spike protein and its role in virus binding and entry; potentially inhibiting Hsp70 protein (a protein required for folding, which was studied during the Zika outbreak) with drugs such as Apoptozole; harvesting, incorporating, or simulating already naturally occurring in-body anti-viral enzymes such as Viperin ddhCTP (which prevents viruses from copying their genetic material and thus from multiplying); utilizing 3D8 scFv (catalytic enzyme) which has hydrolyzing (breaking up, i.e., cleaving) capabilities and protects the host from multifarious viruses regardless of genomic composition; using Ribonucleases (RNases) (June Byun, S., Yuk, S., Jang, Y. et al.) which represent another antiviral therapy approach to degrading viral RNA genomes. etc., and maybe using saline or magnesia-based medicines to encourage hydrolysis since rNA is hurt by alkaline, and saline (salt water) and magnesia have opposite pH levels to human blood (around 7 pH) which is acidic and where viruses can thrive.
(General Ideas in my head)
Develop Anti-viral Medications which build off studies on Arbidol (NS protein inhibitor used for Influenza), NGI-1 (OST inhibitor to prevent sugar protein connections), Remdesivir, Apoptozole (inhibitor of Hsp70 studied during Zika), or other drugs and develop a vaccine based on a protein expression vector, which forms and secretes a chimeric soluble protein that delivers the viral antigen (MIGAL) based on mRNA (Moderna Therapeutics) that stimulates or introduced harvested naturally occurring anti-viral hydrolyzing enzymes/ribonucleotides such as Viperin (Penn State Study) which produces ddhCTP (which prevents viruses from copying their genetic material and thus from), catalytic antibodies such as 3D8 scFv antibody (National Institute of Animal Science, Rural Development Administration, Korea study) while simultaneously discouraging the proteins vital for viral growth such as inhibiting Hsp70 (Stanford University) and host functions such as oligosaccharyltransferase (OST) complex (attaches sugar molecules to proteins vital for some viruses) (Stanford Unveristy) in order to trigger immune responses to mark the viral code for destruction (Moderna Therapuetics methods).
mRNA coded to (1) encourage Viperin ddCTP to prevent viruses from copying genetic info (2) inhibiting proteins essential for viral protein growths and receptor attachment such as NSC 630668-R/1, VER-155008, MAL3–101, MKT-077 (inhibits breast cancer cells), Pifithrin (potent anti-tumor treatment), and Apoptozole (inhibits Hsp70 studied during Zika) (3) inhibiting OST complexes which attaches sugar molecules to viral proteins and (4) triggering immune responses to mark the viral code for destruction
Using wearable Insulin pumps with Anti-viral medications to monitor the bodies white blood cells and immune system to release the anti-viral serum.
Viperin (ddhCTP enzyme studies against viruses)
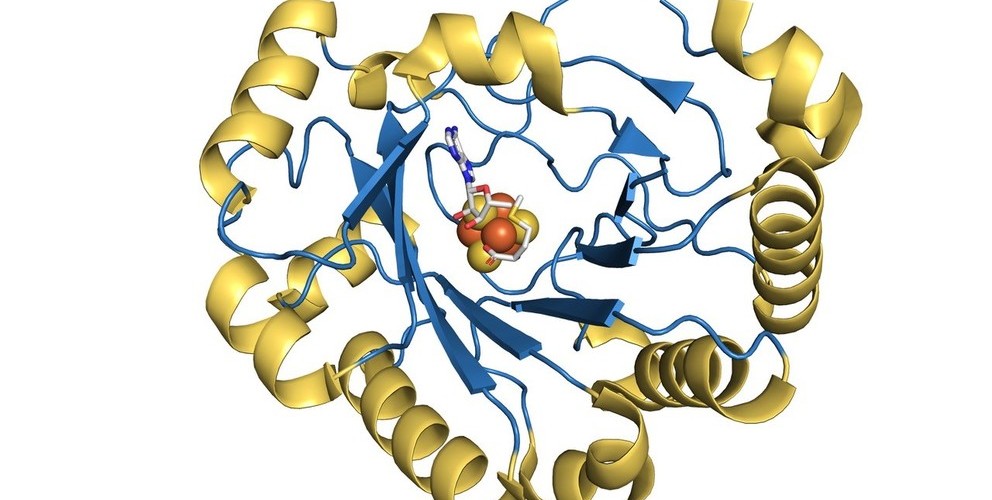
ScienceDaily.com (2018) stated how Penn State scientists in conjunction with researchers at Albert Einstein College of Medicine identified the mode of action of viperin, a naturally occurring enzyme in humans and other mammals that is known to have antiviral effects on viruses such as West Nile, hepatitis C, rabies, and HIV. This discovery could allow researchers to develop a drug that could act as a broad-spectrum therapy for a range of viruses, including Zika. Viperin is the enzyme that facilitates a reaction that produces the molecule ddhCTP, which prevents viruses from copying their genetic material and thus from multiplying. This discovery could allow researchers to develop a drug that induces the human body to produce this molecule and could act as a broad-spectrum therapy for a range of viruses. A virus typically co-opts the host’s genetic building blocks to copy its own genetic material, incorporating molecules called nucleotides into new strands of RNA. The molecule ddhCTP mimics these nucleotide building blocks and becomes incorporated into the virus’s genome. Once incorporated into a new strand of the virus’s RNA, these “nucleotide analogs” prevent an enzyme called RNA polymerase from adding more nucleotides to the strand, thus preventing the virus from making new copies of its genetic material. “Long ago, the paradigm was that in order to kill a virus, you had to kill the infected cell,” said Cameron. “Such a paradigm is of no use when the virus infects an essential cell type with limited capacity for replenishment. The development of nucleotide analogs that function without actually killing the infected cell changed everything.” https://www.sciencedaily.com/releases/2018/06/180620150150.htm
Viperin, a member of the radical S-adenosyl-l-methionine (SAM) superfamily of enzymes, is an interferon-inducible protein implicated in the inhibition of replication of a broad range of RNA and DNA viruses.
Craig Cameron, professor and holder of the Eberly Chair in Biochemistry and Molecular Biology at Penn State and an author of the study. cec@psu.edu
Anthony S. Gizzi, Tyler L. Grove, Jamie J. Arnold, Joyce Jose, Rohit K. Jangra, Scott J. Garforth, Quan Du, Sean M. Cahill, Natalya G. Dulyaninova, James D. Love, Kartik Chandran, Anne R. Bresnick, Craig E. Cameron, Steven C. Almo. A naturally occurring antiviral ribonucleotide encoded by the human genome. Nature, 2018; DOI: 10.1038/s41586-018-0238-4
Penn State. “Compound made inside human body stops viruses from replicating.” ScienceDaily. ScienceDaily, 20 June 2018. www.sciencedaily.com/releases/2018/06/180620150150.htm
[Note: in lieu of antiviral medications, can technology such as wearable Insulin pumps be worn to provide constant or timed doses of anti-viral medications?]
[Note: How are antiviral medications made? It seems by loading antibodies into nanoparticles such as poly [lactic-co-glycolic acid] nanoparticles (3D8-PLGA NPs)]
3D8 scFV Catalytic antibody research in anti-viral research
Korean researchers associated with National Institute of Animal Science, Rural Development Administration; Ajou University School of Medicine, and Sungkyunkwan University, supported by Cooperative Research Program (PJ010201) of the Rural Development Administration of Korea, studied the factors that influence the enzymatic stability of the catalytic antibody 3D8 scFv in various biochemical and physical environments. Based on its enzymatic stability, 3D8 scFv holds good potential for development as an anti-viral therapeutic.
Our study provides an understanding of the factors that influence the enzymatic stability of the catalytic antibody 3D8 scFv in various biochemical and physical environments. Based on its enzymatic stability, 3D8 scFv holds good potential for development as an anti-viral therapeutic.
3D8 scFv is a catalytic antibody with nucleic acid-binding and -hydrolyzing activities that undergoes cellular internalization. Attention has been given to 3D8 scFv due to its anti-viral effect against a broad spectrum of viruses. The anti-viral activity of 3D8 scFv was demonstrated in vitro (Joung et al., 2012, Jun et al., 2010) and in vivo (Lee et al., 2013a, Lee et al., 2013b, Lee et al., 2014) against animal viruses such as classical swine fever virus (CSFV) (Jun et al., 2010), vesicular stomatitis virus (VSV) (Joung et al., 2012, Jun et al., 2010), and human herpes simplex virus-1 (HSV-1) (Lee et al., 2014), as well as against a variety of plant viruses (Lee et al., 2013a, Lee et al., 2013b).
Most recently, the possibility of developing preventive anti-viral probiotics was demonstrated by generating a transgenic Lactobacillus that secretes 3D8 scFv that protected mice against infection by gastrointestinal murine norovirus (Hoang et al., 2015). The mechanism of action of the anti-viral effect of 3D8 scFv is believed to be due to its ability to hydrolyze the nucleic acids (the genome and/or transcripts) of invading viruses during the early phase of infection.
The fact that 3D8 scFv protects the host from multifarious viruses regardless of genomic composition makes 3D8 scFv a candidate for potential application as an anti-viral agent.
https://www.sciencedirect.com/science/article/pii/S0378517315303446
A virus is a small parasite (zombie or android or replicant) that cannot reproduce by itself. Once it infects a susceptible cell, however, a virus can direct the cell machinery to produce more viruses. Most viruses have either RNA or DNA as their genetic material. The nucleic acid may be single- or double-stranded. The entire infectious virus particle, called a virion, consists of the nucleic acid and an outer shell of protein. The simplest viruses contain only enough RNA or DNA to encode four proteins. The most complex can encode 100 – 200 proteins. (https://www.ncbi.nlm.nih.gov/books/NBK21523/)
All viruses utilize normal cellular ribosomes, tRNAs, and translation factors for synthesis of their proteins. Viruses also often express proteins that modify host-cell processes so as to maximize viral replication. (https://www.ncbi.nlm.nih.gov/books/NBK21523/)
Cellular organisms use messenger RNA (mRNA) to convey genetic information (using the nitrogenous bases of guanine, uracil, adenine, and cytosine, denoted by the letters G, U, A, and C) that directs synthesis of specific proteins. Many viruses encode their genetic information using an RNA genome. Some RNA molecules play an active role within cells by catalyzing biological reactions, controlling gene expression, or sensing and communicating responses to cellular signals. One of these active processes is protein synthesis, a universal function in which RNA molecules direct the synthesis of proteins on ribosomes. This process uses transfer RNA (tRNA) molecules to deliver amino acids to the ribosome, where ribosomal RNA (rRNA) then links amino acids together to form coded proteins. https://en.wikipedia.org/wiki/RNA
To understanding how proteins are made, according to ScienceMueseum.org.uk (n.d.) to make a protein, a cell must put a chain of amino acids together in the right order. First, it makes a copy of the relevant DNA instruction in the cell nucleus and takes it into the cytoplasm – a bit like taking a photocopy of the instruction manual from the manager’s office out to the assembly lines in a car factory. Here, the cell decodes the instruction and makes many copies of the protein, which fold into shape as they are produced. (ScienceMueseum.org.uk, n.d.). The first part of the DNA double helix in the cell nucleus unwinds and unzips. The DNA instructions are copied according to ScienceMueseum.org.uk (n.d.) when the DNA instruction is revealed, flanked by ‘start’ and ‘stop’ codes. The cell makes a copy of the DNA in the form of an RNA molecule. The RNA copy is trimmed, and then enters the cell cytoplasm to be decoded. It is now called messenger RNA (mRNA). All proteins are made up of combinations of 20 different amino acids. The RNA code (like DNA) is written in just four different chemical ‘letters’ – bases. The RNA copy made in the cell nucleus is usually much longer than the one that is decoded in the cell cytoplasm. After a cell makes an RNA copy of a gene, it snips out the introns and sticks the exons together to make messenger RNA (mRNA). Once in the cytoplasm, the mRNA is snatched up by tiny protein-assembly machines called ribosomes. Each ribosome works its way along the mRNA, reading the code from ‘start’ to ‘stop’, selecting the correct amino acid building blocks and ejecting a growing protein. It takes just one 50th of a second for the ribosome to select and add each building block. At this rate, a cell can assemble a small protein like insulin in just a few seconds. http://whoami.sciencemuseum.org.uk/whoami/findoutmore/yourbody/whatdoyourcellsdo/howdocellsmakeproteins/howareproteinsmade
Notes: Protons, Electrons, Neutrons > Atoms > Elements (things which expresses same number of protons) > Molecules (Pairings of more than one element, and this categories includes polymers such as RNA and DNA) > Compounds (A combo of multiple molecules) > Substances (Proteins, Enzymes)
RNA is not stable under alkaline conditions unlike DNA. RNA is used to transfer the genetic code from the nucleus to the ribosomes to make proteins. It’s compositions and base sugars are a phosphate backbone, adenine, guanine, cytosine, uracil bases. RNA directly codes for amino acids and acts as a messenger between DNA and ribosomes to make proteins. (Thoughtco.com, Helmenstine, 2020). https://www.thoughtco.com/dna-versus-rna-608191
(Note) So, if many viruses are RNA based (single strand containing sugar phosphate ribose as opposed deoxyribonucleic sugar phosphate) and they can’t exist under alkaline conditions, then does increasing alkaline conditions eradicate or destabilize the virus? If it has a phosphate backbone, can you “break” it’s back, potentially with alkaline? Such as using alkaline hydrolysis.
RNA hydrolysis is a reaction in which a phosphodiester bond in the sugar-phosphate backbone of RNA is broken, cleaving the RNA molecule. RNA hydrolysis occurs when the deprotonated 2’ OH of the ribose, acting as a nucleophile, attacks the adjacent phosphorus in the phosphodiester bond of the sugar-phosphate backbone of the RNA. When the RNA is double-stranded or involved in nucleotide base pairing, it is more stable and spontaneous cleavage is significantly less likely. In these instances, cleavage is done using catalytic enzymes. https://en.wikipedia.org/wiki/RNA_hydrolysis
(Note) Human blood has a Ph level of 7.35 to 7.45. A base on PAR with this (not that I’m saying this would be effective at all) is pure water at around 7, with Saltwater at 8, Baking Soda at 9, and Milk of Magnesia at 10. So, if virus thrive in acid environments such as blood, but they’re adverse to alkaline then does utilizing saline solutions or magnesia-based solutions help anti-viral medications or in anti-viral medication routines, i.e., taking saline or magnesia supplements after talking antiviral medications (those utilizing mRNA or encouraging anti-viral enzyme ribonucleotide production)?
Extreme alkaline and heat treatments to kill viruses as studied by Lemire, Rodriguez, and McIntosh (2016) of the Foreign Animal Disease Diagnostic Laboratory. Note: The only problem with this is that extreme alkaline and heat treatments can’t be performed inside the body, however alkaline is a base, and certain bases such as saline, magnesia, and even baking soda aren’t toxic to the human body in limited doses. Yet, alkaline hydrolysis could be used in spraying techniques for contaminated surfaces in public areas.
Lemire, K.A., Rodriguez, Y.Y. & McIntosh, M.T. (2016) concluded that Treated DNA, while denatured, remains suitable for most common molecular biology procedures including PCR, transformation of E. coli, and molecular sequencing. The procedure ensures not only the inactivation of a variety of viruses but also the degradation through hydrolysis of potentially contaminating infectious + ssRNA viral genomes. Further, their results determined that the new procedure reduced intact RNA beyond the limit of detection by real-time RT-PCR and inactivated viruses by in vitro culture infectivity assays. The authors also state the hardships in research by stating that diagnostics and research of high-consequence animal disease agents is often limited to laboratories with a high level of biosecurity that restrict the transport of biological material. Often, sharing of DNA with external partners is needed to support diagnostics, forensics, or research.
RNA and DNA are differentially susceptible to enzymatic degradations; however, such procedures are susceptible to unintended DNA damage and/or failure due to enzyme or cofactor instabilities. Therefore, we describe the development and verification of a robust and simple chemical and physical method to selectively degrade RNA from purified DNA preparations. The procedure employs incubation of DNA in 0.25 N sodium hydroxide at 65 °C for 1 h followed by neutralization and boiling for 10 min to hydrolyze contaminating RNA and inactivate animal disease viruses from DNA preparations. Additional critical quality control elements include use of a synthetic control RNA (SCR) and an SCR-specific real-time RT-PCR to track effectiveness of the procedure in a parallel treated control sample, and a pH check of reagents to ensure proper neutralization of alkaline conditions.
RNA is uniquely unstable in alkaline conditions because bases can easily deprotonate the hydrogen from the hydroxyl group on the 2’-carbon atom (Fig. 1). This deprotonation causes the oxygen to become negatively charged leading to a nucleophilic attack on the adjacent phosphate atom leading to the cleavage of the phosphopentose backbone of RNA.
This autocatalytic degradation is a property of the RNA phosphopentose backbone itself, the use of alkaline pH combined with heat treatment has been used to selectively degrade RNA from RNA:DNA or RNA:cDNA hybrids
Lemire, K.A., Rodriguez, Y.Y. & McIntosh, M.T. Alkaline hydrolysis to remove potentially infectious viral RNA contaminants from DNA. Virol J 13, 88 (2016). https://doi.org/10.1186/s12985-016-0552-0
Relating to rRNA Hydrolysis, Delehanty, Stuart, Knight, Goldman, Thach, Bongard, & Chang (2005)
(Notes on the Zika Virus)
Huber (2017) interviewed Judith Frydman, PhD, senior author of the paper and a professor of genetics and of biology at Stanford relating to proteins and Zika. Per the articles by Huber (2017), one of those factors is a type of protein called Hsp70, which helps proteins fold correctly and performs a wide range of housekeeping and quality-control functions in cells (Huber, 2019). Based on a series of experiments in mosquito and human cells, the Stanford study found that certain Hsp70 proteins are required in multiple steps of the Zika virus’ lifecycle. By blocking Hsp70 with an Hsp70 inhibitor drug, the researchers were able to prevent virus replication, as recently reported in Cell Reports. “The virus has a much higher demand for Hsp70 than the host cellular processes,” Frydman said. “We can exploit the viral ‘addiction’ to Hsp70 for treatment to prevent the virus from producing the proteins it needs to replicate and infect cells. But most importantly, we show Hsp70 inhibitors can be administered to animals at therapeutically effective doses. To my knowledge, this is the first drug that actually works for Zika-infected animals, protecting them from lethal infection and disease symptoms.” (Huber, 2019). https://scopeblog.stanford.edu/2019/02/06/blocking-zika-new-antiviral-may-treat-and-prevent-infection-a-stanford-study-suggests/
Taguwa et al. (2019) stated we find that for ZIKV, similar to DENV virus, Hsp70 functions at distinct steps of the ZIKV life cycle: virus entry, formation of the active replication complex, and particle production. (Taguwa, Yeh, Rainbolt, Gestwicki, Andino, & Frydman, 2019). https://www.cell.com/cell-reports/pdf/S2211-1247(18)32055-2.pdf
What drugs inhibit Hsp70 current inhibitors identified to target mammalian Hsp70 include NSC 630668-R/1, VER-155008, MAL3–101, MKT-077, Pifithrin, and Apoptozole. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6642669/
How do you find inhibitors? Haystead (2018) “fluorescent-linked enzyme chemoproteomic strategy (FLECS)” “FLECS is a Universal assay that enables purine utilizing proteins of diverse function to be screened against small molecule libraries.” “The FLECS screen is a variation of proteome mining technology utilized in the discovery of the Hsp90 inhibitor SNX5422 21. Proteome mining was designed to screen all purine binding proteins (the purinome) expressed in cells/tissues en masse against large directed chemical libraries, matching early chemical starting points with targets 22,23. The power of this approach enabled not only hundreds of diverse enzymes to be screened at a single step but also enabled the selectivity of a particular hit molecule to be determined simultaneously. The same assay could also then be used in subsequent iterative campaigns to monitor or improve selectivity as one strived to improve the molecules potency and bioavailability.” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6642669/
Yet, HsP70 inhibitors could have effects on renal (kidney) functions.
Haystead (2018) Like NSC 630668-R/1, MAL3–101 is quite large and has a number of labile ester groups. MKT-077 targets the NBD and inhibits proliferation in tumor cell lines, however, severe renal dysfunction in patients was observed in phase I clinical trials. Pifithrin, binds to the SBD of both Hsc70 and Hsp70i disrupting client protein interaction in vitro. In tumor cells the molecule promotes caspase dependent cell death in tumor cells only suggesting it has some specificity to Hsp70i in vivo, although p53 binding has also been shown, which could explain its antitumor actions 18. MKT-O77 and Pifithrin have potential reactive groups that render them covalent modifiers, which may contribute to side effects in vivo. More promising inhibitors of the Hsp70 class are second and 3rd generation MKT-077 analogs JG18 and JG40. Their mechanism of action and specificity lies in interaction with Hsp70 co-chaperones such as NEF https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6642669/
Haystead T. (2018). Fluorescent-Linked Enzyme Chemoproteomic Strategy (FLECS) for Identifying HSP70 Inhibitors. Methods in molecular biology (Clifton, N.J.), 1709, 75–86. https://doi.org/10.1007/978-1-4939-7477-1_6 [Note: NIH grants R01-AI089526-04 to T.A.J.H. and a Department of Defense Transformative Vision Award to T.A.J H.]
Relating to simple sugar’s relation to viruses, Huber (2017) detailed a Stanford and Yale research team who studied OST and its relation to viruses such as Zika. Specifically, they demonstrated the importance of the oligosaccharyltransferase (OST) complex that attaches sugar molecules to proteins. They found flaviviruses did not infect their genetically engineered cells without OST.In the new study, published today in Cell Reports, the Stanford researchers collaborated with scientists at Yale University to test the effectiveness of a drug called NGI-1, which inhibits the activity of the OST complex. They showed that low concentrations of NGI-1 could be used to block the viruses from replicating without harming the host cells — successfully reducing the infection by 99 percent when treating cells immediately after they were infected by Zika or dengue virus, and by 80 percent when administered 24 hours after infection. Their study also indicated that the viruses are unlikely to become resistant to NGI-1. “When you target a host function rather than a viral protein, it’s usually much more difficult for a virus to develop resistance,” Carette said in the release. So, instead of traditional approach of attacking an individual virus directly, the researchers focused on the cellular factors of their human hosts that are essential to many viral infections. (Huber, 2017)
The Cell Report’s (2017) publication of the study spoken about by Huber (2017) conducted by A.S. Puschnik, C.D. Marceau, Y.S. Ooi, K. Majzoub, N. Rinis, J.N. Contessa, and J.E. Carette (2017) stated NGI-1 is an aminobenzamide-sulfonamide compound that targets both OST isoforms and therefore may exhibit antiviral activity against flaviviruses (Figure 1A). To test its inhibitory properties, we infected HEK293 cells with luciferase expressing DENV or ZIKV, treated cells with increasing concentrations of NGI-1, and measured viral replication 48 hr post-infection (hpi) (Figures 1B and 1C). The work was funded in part by the NIH (grants DP2 AI104557 and U19 AI109662 to J.E.C. and RO1CA172391 to J.N.C.), the David and Lucile Packard Foundation (J.E.C.), a Stanford graduate fellowship (A.S.P.), Boehringer Ingelheim Fonds (A.S.P.), and the NSF-GFRP (C.D.M.).
https://www.cell.com/cell-reports/fulltext/S2211-1247(17)31706-0
Fink, S.L., Vojtech, L., Wagoner, J. et al (2018) stated that Arbidol (ARB, umifenovir), used clinically for decades in several countries as an anti-influenza virus drug, inhibits many other viruses. Further, Fink, S.L., Vojtech, L., Wagoner, J. et al (2018) stated that Zika is a distant relative of the hepatitis C virus, which lies in a novel genus within the Flaviviridae called Hepacivirus.
Upon binding to one or more cell-surface receptors, flaviviruses enter cells via endocytosis (note: the taking in of matter by a living cell by invagination [cell eating or drinking, often by encircling) of its membrane to form a vacuole [compartments], i.e., the cell membrane encircles the foreign entity and stores it in a pouch in its inner cell). Once the endosomal lumen is acidified, the viral surface glycoproteins undergo a conformational change, which induces fusion of the endosomal membrane with the viral envelope, releasing the viral genome into the cytoplasm (Fink, S.L., Vojtech, L., Wagoner, J. et al, 2018).
The ZIKV genome is single stranded, positive sense RNA and over 10 kb (kilobase) in length. Like all flaviviruses, the RNA genome encodes a genome-length polyprotein precursor, with proteins arranged in the following order: 5′-C-prM-E-NS1-NS2A-NS2B-NS3-NS4A-NS4B-NS5-3′. The polyprotein is cleaved (split and divided) by cellular and viral proteases (an enzyme that catalyzes [increases the rate of] proteolysis), the breakdown of proteins into smaller polypeptides or single amino acids) to yield the viral structural and non-structural proteins (NS) (Fink, S.L., Vojtech, L., Wagoner, J. et al, 2018). Structural proteins include Capsid (C), precursor Membrane (prM), Envelope (E), and Non-Structural proteins (NS). Many NS proteins have roles in viral replication and include NS1 (modulates host immunity), NS2A, NS2B, NS3 (protease), NS4A, NS4B, NS5 (polymerase) (Fink, S.L., Vojtech, L., Wagoner, J. et al, 2018).
Lastly, Fink, S.L., Vojtech, L., Wagoner, J. et al, (2018), Arbidol (ARB, also known as Umifenovir, PubChem CID 131410), is a synthetic antiviral drug developed 30 years ago to combat seasonal influenza virus11. Since that time, ARB has been shown to inhibit viruses from many different families including orthomyxo12, paramyxo13, picorna14, bunya15, rhabdo16, reo13, toga17, hepadna18, hepaci11,19,20,21,22, and filoviridae23. https://www.nature.com/articles/s41598-018-27224-4
Park (2020) stated that National Institute of Health scientists were studying Remdesivir, a drug, in order to combat the coronavirus. Remdesivir (developed from Ebola) showed encouraging results among animals infected with two related coronaviruses, one responsible for severe acute respiratory syndrome (SARS) and another for causing Middle East respiratory syndrome (MERS).
Yet, instead of Remdesivir, Park (2020) stated that Moderna Therapuetics vaccine is packed with mRNA, the genetic material that comes from DNA and makes proteins. Moderna loads its vaccine with mRNA (note: messenger RNA) that codes for the right coronavirus proteins which then get injected into the body. Immune cells in the lymph nodes can process that mRNA and start making the protein in just the right way for other immune cells to recognize and mark them for destruction, and, Dr Stephen Hodge (President of Moderna) elaborated on the mRNA by stating, “mRNA is really like a software molecule in biology. So our vaccine is like the software program to the body, which then goes and makes the [viral] proteins that can generate an immune response.” https://time.com/5790545/first-covid-19-vaccine/
MIGAL (Galile Research Institute) is developing a coronavirus anti-virus based on its studies into Infectious Bronchitis Virus (IBV). Jaffe-Hoffman (2020) quotes, Dr. Chen Katz, MIGAL’s biotechnology group lead, who stated, the scientific framework for the vaccine is based on a new protein expression vector, which forms and secretes a chimeric soluble protein that delivers the viral antigen into mucosal tissues by self-activated endocytosis, causing the body to form antibodies against the virus.





An expression vector, otherwise known as an expression construct, is usually a plasmid or virus designed for gene expression in cells. The vector is used to introduce a specific gene into a target cell, and can commandeer the cell’s mechanism for protein synthesis to produce the protein encoded by the gene. Expression vectors are the basic tools in biotechnology for the production of proteins.
Polymerase chain reaction (PCR) is a method widely used in molecular biology to rapidly make millions to billions of copies of a specific DNA sample allowing scientists to take a very small sample of DNA and amplify it to a large enough amount to study in detail.
The thermal cycler (also known as a thermocycler, PCR machine or DNA amplifier) is a laboratory apparatus most commonly used to amplify segments of DNA via the polymerase chain reaction (PCR).[1] Thermal cyclers may also be used in laboratories to facilitate other temperature-sensitive reactions, including restriction enzyme digestion or rapid diagnostics.[2] The device has a thermal block with holes where tubes holding the reaction mixtures can be inserted. The cycler then raises and lowers the temperature of the block in discrete, pre-programmed steps.
Flow Cytometry In this process, a sample containing cells or particles is suspended in a fluid and injected into the flow cytometer instrument. The sample is focused to ideally flow one cell at a time through a laser beam, where the light scattered is characteristic to the cells and their components. Cells are often labeled with fluorescent markers, so light is absorbed and then emitted in a band of wavelengths. Tens of thousands of cells can be quickly examined, and the data gathered are processed by a computer. Cell counting, Cell sorting, Determining cell characteristics and function, Detecting microorganisms, Biomarker detection, Protein engineering detection, Diagnosis of health disorders such as blood cancers
[Note: seems similar to Fluorescent-Linked Enzyme Chemoproteomic Strategy by TAJ Haystead]
An assay is an investigative (analytic) procedure in laboratory medicine, pharmacology, environmental biology and molecular biology for qualitatively assessing or quantitatively measuring the presence, amount, or functional activity of a target entity (the analyte). The analyte can be a drug, biochemical substance, or cell in an organism or organic sample.[1][2] The measured entity is often called the analyte, the measurand, or the target of the assay. An assay usually aims to measure an analyte’s intensive property and express it in the relevant measurement unit (e.g. molarity, density, functional activity in enzyme international units, degree of effect in comparison to a standard, etc.).
An enzyme-linked immunosorbent assay, also called ELISA or EIA, is a test that detects and measures antibodies in your blood. This test can be used to determine if you have antibodies related to certain infectious conditions.
Recirculating chillers deliver a flow of temperature-controlled fluid. They are enclosed circulators featuring robust cooling systems used for demanding continuous-use lab applications.
A vortex mixer, or vortexer, is a simple device used commonly in laboratories to mix small vials of liquid. Vortex mixers are quite commonplace in bioscience laboratories. In cell culture and microbiology laboratories they may be used to suspend cells. In a biochemical or analytical laboratory they may be used to mix the reagents of an assay or to mix an experimental sample and a dilutant.
An antibody (Ab), also known as an immunoglobulin (Ig), is a large, Y-shaped protein produced mainly by plasma cells that is used by the immune system to neutralize pathogens such as pathogenic bacteria and viruses.
Notes on Labs, Universities associated with Projects, Equipment Suppliers, and Organizations:
- Moderna Therapuetics https://www.modernatx.com/
- Sino Biological https://www.sinobiological.com/
- Promega https://www.promega.com/
- BD Bioscience https://www.bdbiosciences.com/en-us
- ThermoFisher (includes Life Technologies and Fisher Scientific) https://www.thermofisher.com/us/en/home.html
- JEOL https://www.jeolusa.com/
- Qiagen https://www.qiagen.com/us/
- Agilent https://www.agilent.com/
- VectorLabs https://vectorlabs.com/
- Mettler Toledo https://www.mt.com/us/en/home.html
- Hanna Instruments https://www.hannainst.com/
- Intertek https://www.intertek.com/
- Sigma Aldrich https://www.sigmaaldrich.com/united-states.html
- Penn State University on Viperin Antiviral Enzyme Research http://www.cameronlab.com/ cec@psu.edu
- Albert Einstein College of Medicine on Antiviral Viperin Enzymes Research
- Stanford University on Exploiting Zika addiction to Hsp70 protein jfrydman@stanford.edu
- Stanford University of the OST Zika Research carette@stanford.edu
- University of Arizona on Nanotechnology
- National Institute of Animal Science, Rural Development Administration; Ajou University School of Medicine, and Sungkyunkwan University on 3D8 research
- Rockefeller University
- National Institute of Health on Remdesivir
- MIGAL (Gallile Research Institute) on IBV research and protein expression vectors
- Reagent and Vaccine Services Section (RVSS) of the USDA National Veterinary Services Laboratories’ (NVSL) FADDL located at the Plum Island Animal Disease Center (PIADC) relating to Alkaline Hydrolysis and Heat Treatment to kill viruses
Equipment used for the OST NSI-1 Zika Study by the Stanford Research and Yale Team
- Renilla Luciferase Assay system (Promega) for Luciferase expression measurement
- 96-well plates https://www.promega.com/products/luciferase-assays/reporter-assays/renilla-luciferase-assay-system/?catNum=E2810
- CellTiter-Glo (Promega) for Cell Growth in Culture Measurement https://www.promega.com/products/cell-health-assays/cell-viability-and-cytotoxicity-assays/celltiter_glo-luminescent-cell-viability-assay/?catNum=G7570
- Trypan blue solution for Cell Growth Measurement
- Bio-Rad Gene Pulser Xcell electroporator use for electroporation techniques in which an electrical field is applied to cells in order to increase the permeability of the cell membrane, allowing chemicals, drugs, or DNA to be introduced into the cell (also called electrotransfer) https://www.bio-rad.com/en-us/product/gene-pulser-xcell-electroporation-systems?ID=b1a35eb3-d55c-47b3-aaf3-95e4d1d85848
- Perm/Wash buffer (BD Biosciences) Cell permeabilization Perm/Wash buffer (BD Biosciences) https://www.bdbiosciences.com/us/applications/research/intracellular-flow/intracellular-buffers-and-ancillary-reagents/permwash-buffer/p/554723
- IgG Alexa 488 (Life Technologies) for Incubation
- FlowJo 9 for data analysis for Flow Cytometry http://v9docs.flowjo.com/html/index.html
- GraphPad Prism 7 for Statistical Analysis https://www.graphpad.com/scientific-software/prism/
Equipment used by the Foreign Animal Disease Diagnostic Laboratory team (Lemire, Rodriguez, and McIntosh) when studying Heat Treatment and Alkaline Hydrolysis in killing viruses:
- RNeasy® Mini Kit (Qiagen, Valencia, CA, USA) for RNA Extractions https://www.qiagen.com/us/products/discovery-and-translational-research/dna-rna-purification/rna-purification/total-rna/rneasy-mini-kit/#orderinginformation
- DNeasy® Blood and Tissue Kit (Qiagen, Valencia, CA, USA) for DNA Extractions https://www.qiagen.com/us/products/discovery-and-translational-research/dna-rna-purification/dna-purification/genomic-dna/dneasy-blood-and-tissue-kit/#orderinginformation
- Qiagen QIAquick Spin Miniprep PCR Purification Kit for Plasmid Purification https://www.qiagen.com/us/products/qiaquick-pcr-purification-kit/#orderinginformation
- pBlueScript II plasmid (Agilent Technologies) Plasmid Propagation https://www.agilent.com/en/product/mutagenesis-cloning/cloning-vectors-kits/cloning-vector-kits/pbluescript-ii-vectors-233066
- pGEM-T Easy vector plasmid (Promega Corp.) for PCR and Plasmid Propagation https://www.promega.com/products/pcr/pcr-cloning/pgem-t-easy-vector-systems/?catNum=A1360
- QIAquick® PCR Purification kit (Qiagen) for Purification Reverse-transcriptase polymerase chain reaction PCR https://www.qiagen.com/us/products/qiaquick-pcr-purification-kit/#orderinginformation
- VECTASTAIN® ABC-AP KIT. Viral Isolation Straining https://vectorlabs.com/vectastain-abc-ap-kit-standard-x123.html
- PRIMARIA™ coated culture flasks https://www.fishersci.com/shop/products/corning-primaria-tissue-culture-dishes-3/p-176159
- Spearman-Kärber method was used to calculate titrations
- NanoDrop 2000 spectrophotometer (Fisher Scientific, Pittsburg, PA, USA) for DNA quantification https://www.thermofisher.com/order/catalog/product/ND-2000#/ND-2000
- Sequencher® software (Gene Codes) for DNA Contig Sequencing https://www.genecodes.com/
- Kingfisher 96 magnetic particle processor (Thermo Fisher Scientific) Sequencing product purification, contaminant and impurity QC testing https://www.thermofisher.com/us/en/home/life-science/bioproduction/contaminant-and-impurity-testing/sample-prep-and-automation/kingfisher-flex-magnetic-particle-processor.html
- 3730XL DNA sequencer (Applied Biosystems) for Nucleotide Sequencing
- Invitrogen-Thermo Fisher Scientific https://www.thermofisher.com/us/en/home/brands/invitrogen.html
- GeneAmp® EZ rTth RNA PCR kit (Life Technologies, Grand Island, NY, USA)
RNA Sample Amplification:
- SmartCycler II (Cepheid, Sunnyvale, CA) with automatic background subtraction on
- TaqMan® Fast Virus 1-Step Master Mix (Life Technologies, Grand Island, NY, USA) Applied Biosystems® 7500 Real-Time PCR System (Life Technologies, Grand Island, NY, USA). https://www.thermofisher.com/order/catalog/product/4351106?SID=srch-srp-4351106#/4351106?SID=srch-srp-4351106
- Path-ID™ Multiplex One-Step Kit (Life Technologies, Grand Island, NY, USA) https://www.thermofisher.com/order/catalog/product/4442135#/4442135
DNA Sample Amplification:
- SmartCycler II PCR platform (Cepheid, Sunnyvale, CA, USA)
- TaqMan® EZ RT-PCR kit (Life Technologies, Grand Island, NY, USA). https://www.thermofisher.com/order/catalog/product/A28527?SID=srch-srp-A28527#/A28527?SID=srch-srp-A28527
https://time.com/5790545/first-covid-19-vaccine/
https://www.nature.com/articles/s41598-018-27224-4
https://en.wikipedia.org/wiki/RNA_hydrolysis
https://en.wikipedia.org/wiki/Gene_delivery#RNA-based_viral_vectors
https://asunow.asu.edu/20180212-discoveries-cancer-fighting-nanorobots-seek-and-destroy-tumors